The global burden of lung cancer: current status and future trends
Lung cancer is the leading cause of cancer-related death worldwide. However, lung cancer incidence and mortality rates differ substantially across the world, reflecting varying patterns of tobacco smoking, exposure to environmental risk factors and genetics. Tobacco smoking is the leading risk factor for lung cancer. Lung cancer incidence largely reflects trends in smoking patterns, which generally vary by sex and economic development. For this reason, tobacco control campaigns are a central part of global strategies designed to reduce lung cancer mortality. Environmental and occupational lung cancer risk factors, such as unprocessed biomass fuels, asbestos, arsenic and radon, can also contribute to lung cancer incidence in certain parts of the world. Over the past decade, large-cohort clinical studies have established that low-dose CT screening reduces lung cancer mortality, largely owing to increased diagnosis and treatment at earlier disease stages. These data have led to recommendations that individuals with a high risk of lung cancer undergo screening in several economically developed countries and increased implementation of screening worldwide. In this Review, we provide an overview of the global epidemiology of lung cancer. Lung cancer risk factors and global risk reduction efforts are also discussed. Finally, we summarize lung cancer screening policies and their implementation worldwide.
Key points
- Lung cancer is the leading cause of cancer death globally, with incidence and mortality trends varying greatly by country and largely reflecting differences in tobacco smoking trends.
- Cigarette smoking is the most prevalent lung cancer risk factor, although environmental exposures, such as biomass fuels, asbestos, arsenic and radon, are all important lung factor risk factors with levels of exposure that vary widely across the globe.
- Lung cancer incidence and mortality rates are highest in economically developed countries in which tobacco smoking peaked several decades ago, although these rates have mostly now peaked and are declining.
- Reductions in lung cancer mortality in economically developed countries reflect decreased incidence (mirroring declines in tobacco smoking) and improvements in treatment of patients with advanced-stage disease, including immunotherapies and targeted therapies.
- In low-income and middle-income countries at the later stages of the tobacco epidemic, both lung cancer incidence and mortality are increasing, thus highlighting the importance of tobacco mitigation policies for reducing the global burden of lung cancer.
- Low-dose CT-based lung cancer screening reduces lung cancer mortality, although adoption of lung cancer screening programmes has been slow, with limited uptake compared with other cancer screening programmes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Lung cancer in patients who have never smoked — an emerging disease
Article 09 January 2024
Trends in lung cancer incidence by age, sex and histology from 2012 to 2025 in Catalonia (Spain)
Article Open access 02 December 2021

The fraction of lung cancer attributable to smoking in the Norwegian Women and Cancer (NOWAC) Study
Article Open access 27 October 2020
References
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). ArticlePubMedGoogle Scholar
- Travis, W. D., Brambilla, E., Burke, A. P., Marx, A. & Nicholson, A. G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart (IARC, 2015).
- Schabath, M. B. & Cote, M. L. Cancer progress and priorities: lung cancer. Cancer Epidemiol. Biomark. Prev.28, 1563–1579 (2019). ArticleGoogle Scholar
- Lortet-Tieulent, J. et al. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer84, 13–22 (2014). ArticleCASPubMedGoogle Scholar
- Wakelee, H. A. et al. Lung cancer incidence in never smokers. J. Clin. Oncol.25, 472–478 (2007). ArticlePubMedGoogle Scholar
- United Nations Development Programme. Human development report 2021-22. UNDPhttp://report.hdr.undp.org (2022).
- Jemal, A., Ma, J., Rosenberg, P. S., Siegel, R. & Anderson, W. F. Increasing lung cancer death rates among young women in southern and midwestern states. J. Clin. Oncol.30, 2739–2744 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Jemal, A. et al. Higher lung cancer incidence in young women than young men in the united states. N. Engl. J. Med.378, 1999–2009 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Islami, F. et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United states. CA Cancer J. Clin.68, 31–54 (2018). ArticlePubMedGoogle Scholar
- Siegel, D. A., Fedewa, S. A., Henley, S. J., Pollack, L. A. & Jemal, A. Proportion of never smokers among men and women with lung cancer in 7 US states. JAMA Oncol.7, 302–304 (2021). ArticlePubMedGoogle Scholar
- Sakoda, L. C. et al. Trends in smoking-specific lung cancer incidence rates within a US integrated health system, 2007-2018. Chesthttps://doi.org/10.1016/j.chest.2023.03.016 (2023). ArticlePubMedGoogle Scholar
- Pelosof, L. et al. Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. J. Natl Cancer Inst.https://doi.org/10.1093/jnci/djw295 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Meza, R., Meernik, C., Jeon, J. & Cote, M. L. Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS ONE10, e0121323 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Haiman, C. A. et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N. Engl. J. Med.354, 333–342 (2006). ArticleCASPubMedGoogle Scholar
- Murphy, S. E. Biochemistry of nicotine metabolism and its relevance to lung cancer. J. Biol. Chem.296, 100722 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin.71, 7–33 (2021). ArticlePubMedGoogle Scholar
- Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin.72, 7–33 (2022). ArticlePubMedGoogle Scholar
- Howlader, N. et al. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med.383, 640–649 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Singh, G. K. & Jemal, A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J. Environ. Public Health2017, 2819372 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Blom, E. F., Ten Haaf, K., Arenberg, D. A. & de Koning, H. J. Disparities in receiving guideline-concordant treatment for lung cancer in the United States. Ann. Am. Thorac. Soc.17, 186–194 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Sineshaw, H. M. et al. County-level variations in receipt of surgery for early-stage non-small cell lung cancer in the United States. Chest157, 212–222 (2020). ArticlePubMedGoogle Scholar
- GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet397, 2337–2360 (2021). ArticleGoogle Scholar
- Allemani, C. et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet391, 1023–1075 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Jani, C. et al. Lung cancer mortality in Europe and the USA between 2000 and 2017: an observational analysis. ERJ Open. Res.https://doi.org/10.1183/23120541.00311-2021 (2021). ArticlePubMedPubMed CentralGoogle Scholar
- Malvezzi, M. et al. European cancer mortality predictions for the year 2023 with focus on lung cancer. Ann. Oncol.34, 410–419 (2023). ArticleCASPubMedGoogle Scholar
- Carioli, G. et al. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann. Oncol.31, 650–658 (2020). ArticleCASPubMedGoogle Scholar
- Alves, L., Bastos, J. & Lunet, N. Trends in lung cancer mortality in Portugal (1955-2005). Rev. Port. Pneumol.15, 575–587 (2009). ArticlePubMedGoogle Scholar
- Martínez, C., Guydish, J., Robinson, G., Martínez-Sánchez, J. M. & Fernández, E. Assessment of the smoke-free outdoor regulation in the WHO European region. Prev. Med.64, 37–40 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Forsea, A. M. Cancer registries in Europe – going forward is the only option. Ecancermedicalscience10, 641 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Cho, B. C. et al. Genomic landscape of non-small cell lung cancer (NSCLC) in East Asia using circulating tumor DNA (ctDNA) in clinical practice. Curr. Oncol.29, 2154–2164 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Mathias, C. et al. Lung cancer in Brazil. J. Thorac. Oncol.15, 170–175 (2020). ArticlePubMedGoogle Scholar
- Souza, M. C., Vasconcelos, A. G. & Cruz, O. G. Trends in lung cancer mortality in Brazil from the 1980s into the early 21st century: age-period-cohort analysis. Cad. Saude Publica28, 21–30 (2012). ArticlePubMedGoogle Scholar
- Jiang, D. et al. Trends in cancer mortality in China from 2004 to 2018: a nationwide longitudinal study. Cancer Commun.41, 1024–1036 (2021). ArticleGoogle Scholar
- Parascandola, M. & Xiao, L. Tobacco and the lung cancer epidemic in China. Transl. Lung Cancer Res.8, S21–S30 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Hosgood, H. D. 3rd et al. In-home coal and wood use and lung cancer risk: a pooled analysis of the International Lung Cancer Consortium. Env. Health Perspect.118, 1743–1747 (2010). ArticleCASGoogle Scholar
- Kurmi, O. P., Arya, P. H., Lam, K. B., Sorahan, T. & Ayres, J. G. Lung cancer risk and solid fuel smoke exposure: a systematic review and meta-analysis. Eur. Respir. J.40, 1228–1237 (2012). ArticlePubMedGoogle Scholar
- Qiu, A. Y., Leng, S., McCormack, M., Peden, D. B. & Sood, A. Lung effects of household air pollution. J. Allergy Clin. Immunol. Pract.10, 2807–2819 (2022). ArticleCASPubMedGoogle Scholar
- Zhang, M. et al. Trends in smoking prevalence in urban and rural China, 2007 to 2018: findings from 5 consecutive nationally representative cross-sectional surveys. PLoS Med.19, e1004064 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Pineros, M., Znaor, A., Mery, L. & Bray, F. A global cancer surveillance framework within noncommunicable disease surveillance: making the case for population-based cancer registries. Epidemiol. Rev.39, 161–169 (2017). ArticlePubMedGoogle Scholar
- Wei, W. et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol.21, e342–e349 (2020). ArticlePubMedGoogle Scholar
- Mathur, P. et al. Cancer statistics, 2020: report from National Cancer Registry Programme, India. JCO Glob. Oncol.6, 1063–1075 (2020). ArticlePubMedGoogle Scholar
- Nath, A., Sathishkumar, K., Das, P., Sudarshan, K. L. & Mathur, P. A clinicoepidemiological profile of lung cancers in India – results from the National Cancer Registry Programme. Indian J. Med. Res.155, 264–272 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Singh, N. et al. Lung cancer in India. J. Thorac. Oncol.16, 1250–1266 (2021). ArticlePubMedGoogle Scholar
- Kaur, H. et al. Evolving epidemiology of lung cancer in India: reducing non-small cell lung cancer–not otherwise specified and quantifying tobacco smoke exposure are the key. Indian J. Cancer54, 285–290 (2017). ArticleCASPubMedGoogle Scholar
- Mohan, A. et al. Clinical profile of lung cancer in North India: a 10-year analysis of 1862 patients from a tertiary care center. Lung India37, 190–197 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Shaikh, R., Janssen, F. & Vogt, T. The progression of the tobacco epidemic in India on the national and regional level, 1998-2016. BMC Public Health22, 317 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- India State-Level Disease Burden Initiative Cancer Collaborators. The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Oncol.19, 1289–1306 (2022). Google Scholar
- & Piñeros, M. et al. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg. Health Am.13, 100294 (2022). PubMedPubMed CentralGoogle Scholar
- Raez, L. E. et al. The burden of lung cancer in Latin-America and challenges in the access to genomic profiling, immunotherapy and targeted treatments. Lung Cancer119, 7–13 (2018). ArticlePubMedGoogle Scholar
- Pakzad, R., Mohammadian-Hafshejani, A., Ghoncheh, M., Pakzad, I. & Salehiniya, H. The incidence and mortality of lung cancer and their relationship to development in Asia. Transl. Lung Cancer Res.4, 763–774 (2015). PubMedPubMed CentralGoogle Scholar
- Hamdi, Y. et al. Cancer in Africa: the untold story. Front. Oncol.11, 650117 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Centers for Disease Control and Prevention. What are the risk factors for lung cancer? CDChttps://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm (2022).
- Peto, R. et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. Br. Med. J.321, 323–329 (2000). ArticleCASGoogle Scholar
- Boffetta, P. et al. Cigar and pipe smoking and lung cancer risk: a multicenter study from Europe. J. Natl Cancer Inst.91, 697–701 (1999). ArticleCASPubMedGoogle Scholar
- Pednekar, M. S., Gupta, P. C., Yeole, B. B. & Hébert, J. R. Association of tobacco habits, including bidi smoking, with overall and site-specific cancer incidence: results from the Mumbai cohort study. Cancer Causes Control.22, 859–868 (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Proctor, R. N. The history of the discovery of the cigarette–lung cancer link: evidentiary traditions, corporate denial, global toll. Tob. Control.21, 87–91 (2012). ArticlePubMedGoogle Scholar
- Doll, R. & Hill, A. B. The mortality of doctors in relation to their smoking habits. Br. Med. J.1, 1451 (1954). ArticleCASPubMedPubMed CentralGoogle Scholar
- US Department of Health, Education, and Welfare. Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service (US Public Health Service, 1964).
- Brawley, O. W., Glynn, T. J., Khuri, F. R., Wender, R. C. & Seffrin, J. R. The first Surgeon General’s report on smoking and health: the 50th anniversary. CA Cancer J. Clin.64, 5–8 (2014). ArticlePubMedGoogle Scholar
- WHO Framework Convention on Tobacco Control. 2021 global progress report on implementation of the WHO Framework Convention on Tobacco Control (WHO FCTC, 2022).
- Oberg, M., Jaakkola, M. S., Woodward, A., Peruga, A. & Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet377, 139–146 (2011). ArticlePubMedGoogle Scholar
- Office on Smoking and Health. The Health Consequences of Involuntary Exposure to Tobacco Smoke: a Report of the Surgeon General (Centers for Disease Control and Prevention, 2006).
- Yousuf, H. et al. Estimated worldwide mortality attributed to secondhand tobacco smoke exposure, 1990-2016. JAMA Netw. Open3, e201177 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Bracken-Clarke, D. et al. Vaping and lung cancer – a review of current data and recommendations. Lung Cancer153, 11–20 (2021). ArticlePubMedGoogle Scholar
- Centers for Disease Control and Prevention. Trends in tobacco use among youth. CDChttps://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/trends-in-tobacco-use-among-youth.html (2022).
- Sindelar, J. L. Regulating vaping – policies, possibilities, and perils. N. Engl. J. Med.382, e54 (2020). ArticlePubMedGoogle Scholar
- Campus, B., Fafard, P., St Pierre, J. & Hoffman, S. J. Comparing the regulation and incentivization of e-cigarettes across 97 countries. Soc. Sci. Med.291, 114187 (2021). ArticlePubMedGoogle Scholar
- Bruce, N. et al. Does household use of biomass fuel cause lung cancer? A systematic review and evaluation of the evidence for the GBD 2010 study. Thorax70, 433–441 (2015). ArticlePubMedGoogle Scholar
- Woolley, K. E. et al. Effectiveness of interventions to reduce household air pollution from solid biomass fuels and improve maternal and child health outcomes in low- and middle-income countries: a systematic review protocol. Syst. Rev.10, 33 (2021). ArticlePubMedPubMed CentralGoogle Scholar
- Johnston, F. H. et al. Estimated global mortality attributable to smoke from landscape fires. Environ. Health Perspect.120, 695–701 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Korsiak, J. et al. Long-term exposure to wildfires and cancer incidence in Canada: a population-based observational cohort study. Lancet Planet. Health6, e400–e409 (2022). ArticlePubMedGoogle Scholar
- Rousseau, M.-C., Straif, K. & Siemiatycki, J. IARC carcinogen update. Environ. Health Perspect.113, A580–A581 (2005). ArticlePubMedPubMed CentralGoogle Scholar
- Yuan, T., Zhang, H., Chen, B., Zhang, H. & Tao, S. Association between lung cancer risk and inorganic arsenic concentration in drinking water: a dose-response meta-analysis. Toxicol. Res.7, 1257–1266 (2018). ArticleCASGoogle Scholar
- Shankar, S., Shanker, U. & Shikha Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J.2014, 304524 (2014). ArticleGoogle Scholar
- D’Ippoliti, D. et al. Arsenic in drinking water and mortality for cancer and chronic diseases in central Italy, 1990-2010. PLoS ONE10, e0138182 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Ferdosi, H. et al. Arsenic in drinking water and lung cancer mortality in the United States: an analysis based on US counties and 30 years of observation (1950-1979). J. Environ. Public Health2016, 1602929 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Ferreccio, C. et al. Arsenic, tobacco smoke, and occupation: associations of multiple agents with lung and bladder cancer. Epidemiol24, 898–905 (2013). ArticleGoogle Scholar
- Wu, M. M., Kuo, T. L., Hwang, Y. H. & Chen, C. J. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am. J. Epidemiol.130, 1123–1132 (1989). ArticleCASPubMedGoogle Scholar
- Oberoi, S., Barchowsky, A. & Wu, F. The global burden of disease for skin, lung, and bladder cancer caused by arsenic in food. Cancer Epidemiol. Biomark. Prev.23, 1187–1194 (2014). ArticleCASGoogle Scholar
- UNICEF. Arsenic Primer: Guidance on the Investigation and Mitigation of Arsenic Contamination (UNICEF, 2018).
- Turner, M. C. et al. Radon and lung cancer in the American Cancer Society Cohort. Cancer Epidemiol. Biomark. Prev.20, 438–448 (2011). ArticleCASGoogle Scholar
- Ngoc, L. T. N., Park, D. & Lee, Y. C. Human health impacts of residential radon exposure: updated systematic review and meta-analysis of case-control studies. Int. J. Environ. Res. Public Health20, 97 (2012). ArticleGoogle Scholar
- Shan, X. et al. A global burden assessment of lung cancer attributed to residential radon exposure during 1990-2019. Indoor Air32, e13120 (2022). ArticlePubMedGoogle Scholar
- World Health Organization. WHO Handbook on Indoor Radon: a Public Health Perspective (WHO, 2009).
- US Environmental Protection Agency. The national radon action plan – a strategy for saving lives. EPAhttps://www.epa.gov/radon/national-radon-action-plan-strategy-saving-lives (2023).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens. Part F: Chemical Agents and Related Occupations (IARC, 2012).
- Pira, E., Donato, F., Maida, L. & Discalzi, G. Exposure to asbestos: past, present and future. J. Thorac. Dis.10, S237–S245 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Villeneuve, P. J., Parent, M., Harris, S. A. & Johnson, K. C. Occupational exposure to asbestos and lung cancer in men: evidence from a population-based case-control study in eight Canadian provinces. BMC Cancer12, 595 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Lash, T. L., Crouch, E. A. & Green, L. C. A meta-analysis of the relation between cumulative exposure to asbestos and relative risk of lung cancer. Occup. Environ. Med.54, 254–263 (1997). ArticleCASPubMedPubMed CentralGoogle Scholar
- Nelson, H. H. & Kelsey, K. T. The molecular epidemiology of asbestos and tobacco in lung cancer. Oncogene21, 7284–7288 (2002). ArticleCASPubMedGoogle Scholar
- Markowitz, S. B., Levin, S. M., Miller, A. & Morabia, A. Asbestos, asbestosis, smoking, and lung cancer. New findings from the North American Insulator cohort. Am. J. Respir. Crit. Care Med.188, 90–96 (2013). ArticlePubMedGoogle Scholar
- Mossman B. T., Gualtieri A. F. in Occupational Cancers (eds.Anttila S. & Boffetta P.) 239–256 (Springer, 2020).
- Thives, L. P., Ghisi, E., Thives Júnior, J. J. & Vieira, A. S. Is asbestos still a problem in the world? A current review. J. Environ. Manag.319, 115716 (2022). ArticleGoogle Scholar
- Benbrahim-Tallaa, L. et al. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol.13, 663–664 (2012). ArticlePubMedGoogle Scholar
- Ge, C. et al. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. a pooled exposure-response analysis of 14 case-control studies. Am. J. Respir. Crit. Care Med.202, 402–411 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Garshick, E. et al. Lung cancer and elemental carbon exposure in trucking industry workers. Env. Health Perspect.120, 1301–1306 (2012). ArticleGoogle Scholar
- Silverman, D. T. et al. The diesel exhaust in miners study: a nested case-control study of lung cancer and diesel exhaust. J. Natl Cancer Inst.104, 855–868 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Vermeulen, R. et al. Exposure-response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts. Environ. Health Perspect.122, 172–177 (2024). ArticleGoogle Scholar
- Young, R. P. et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J.34, 380–386 (2009). ArticleCASPubMedGoogle Scholar
- de Torres, J. P. et al. Lung cancer in patients with chronic obstructive pulmonary disease-incidence and predicting factors. Am. J. Respir. Crit. Care Med.184, 913–919 (2011). ArticlePubMedGoogle Scholar
- Durham, A. L. & Adcock, I. M. The relationship between COPD and lung cancer. Lung Cancer90, 121–127 (2015). ArticleCASPubMedGoogle Scholar
- Young, R. P. et al. Individual and cumulative effects of GWAS susceptibility loci in lung cancer: associations after sub-phenotyping for COPD. PLoS ONE6, e16476 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Saber Cherif, L. et al. The nicotinic receptor polymorphism rs16969968 is associated with airway remodeling and inflammatory dysregulation in COPD patients. Cells11, 2937 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sigel, K., Makinson, A. & Thaler, J. Lung cancer in persons with HIV. Curr. Opin. Hiv. AIDS12, 31–38 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Shiels, M. S., Cole, S. R., Mehta, S. H. & Kirk, G. D. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J. Acquir. Immune Defic. Syndr.55, 510–515 (2010). ArticlePubMedPubMed CentralGoogle Scholar
- Sigel, K. et al. HIV as an independent risk factor for incident lung cancer. AIDS26, 1017–1025 (2012). ArticlePubMedGoogle Scholar
- Engels, E. A. et al. Elevated incidence of lung cancer among HIV-infected individuals. J. Clin. Oncol.24, 1383–1388 (2006). ArticlePubMedGoogle Scholar
- Chaturvedi, A. K. et al. Elevated risk of lung cancer among people with AIDS. AIDS21, 207–213 (2007). ArticlePubMedGoogle Scholar
- Kirk, G. D. et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin. Infect. Dis.45, 103–110 (2007). ArticlePubMedGoogle Scholar
- Parker, M. S., Leveno, D. M., Campbell, T. J., Worrell, J. A. & Carozza, S. E. AIDS-related bronchogenic carcinoma: fact or fiction? Chest113, 154–161 (1998). ArticleCASPubMedGoogle Scholar
- Patel, P. et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann. Intern. Med.148, 728–736 (2008). ArticlePubMedGoogle Scholar
- Winstone, T. A., Man, S. F., Hull, M., Montaner, J. S. & Sin, D. D. Epidemic of lung cancer in patients with HIV infection. Chest143, 305–314 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hessol, N. A. et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS29, 1183–1193 (2015). ArticlePubMedGoogle Scholar
- Bearz, A. et al. Lung cancer in HIV positive patients: the GICAT experience. Eur. Rev. Med. Pharmacol. Sci.18, 500–508 (2014). CASPubMedGoogle Scholar
- O’Connor, E. A. et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. J. Am. Med. Assoc.327, 2334–2347 (2022). ArticleGoogle Scholar
- Wei, X. et al. Diet and risk of incident lung cancer: a large prospective cohort study in UK Biobank. Am. J. Clin. Nutr.114, 2043–2051 (2021). ArticlePubMedGoogle Scholar
- Xue, X. J. et al. Red and processed meat consumption and the risk of lung cancer: a dose-response meta-analysis of 33 published studies. Int. J. Clin. Exp. Med.7, 1542–1553 (2014). PubMedPubMed CentralGoogle Scholar
- Vieira, A. R. et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann. Oncol.27, 81–96 (2016). ArticleCASPubMedGoogle Scholar
- Amararathna, M., Johnston, M. R. & Rupasinghe, H. P. V. Plant polyphenols as chemopreventive agents for lung cancer. Int. J. Mol. Sci.17, 1352 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Alsharairi, N. A. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: a review of the literature. Nutrients11, 725 (2016). ArticleGoogle Scholar
- The Lung Cancer Cohort Consortium.Circulating folate, vitamin B6, and methionine in relation to lung cancer risk in the Lung Cancer Cohort Consortium (LC3). J. Natl Cancer Inst.110, 57–67 (2018). ArticleGoogle Scholar
- Slatore, C. G., Littman, A. J., Au, D. H., Satia, J. A. & White, E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am. J. Respir. Crit. Care Med.177, 524–530 (2008). ArticleCASPubMedGoogle Scholar
- Verbeek, J. H. et al. An approach to quantifying the potential importance of residual confounding in systematic reviews of observational studies: a GRADE concept paper. Environ. Int.157, 106868 (2021). ArticlePubMedGoogle Scholar
- Cortés-Jofré, M., Rueda, J. R., Asenjo-Lobos, C., Madrid, E. & Bonfill Cosp, X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst. Rev.3, Cd002141 (2020). PubMedGoogle Scholar
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med.330, 1029–1035 (1994). ArticleGoogle Scholar
- Omenn, G. S. et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med.334, 1150–1155 (1996). ArticleCASPubMedGoogle Scholar
- Pearson-Stuttard, J. et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol.6, e6–e15 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Lennon, H., Sperrin, M., Badrick, E. & Renehan, A. G. The obesity paradox in cancer: a review. Curr. Oncol. Rep.18, 56 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Duan, P. et al. Body mass index and risk of lung cancer: systematic review and dose-response meta-analysis. Sci. Rep.5, 16938 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ardesch, F. H. et al. The obesity paradox in lung cancer: associations with body size versus body shape. Front. Oncol.10, 591110 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yu, D. et al. Overall and central obesity and risk of lung cancer: a pooled analysis. J. Natl Cancer Inst.110, 831–842 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Leiter, A. et al. Assessing the association of diabetes with lung cancer risk. Transl. Lung Cancer Res.10, 4200–4208 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yi, Z. H. et al. Association between diabetes mellitus and lung cancer: meta-analysis. Eur. J. Clin. Invest.50, e13332 (2020). ArticlePubMedGoogle Scholar
- Carreras-Torres, R. et al. Obesity, metabolic factors and risk of different histological types of lung cancer: a Mendelian randomization study. PLoS ONE12, e0177875 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Dziadziuszko, R., Camidge, D. R. & Hirsch, F. R. The insulin-like growth factor pathway in lung cancer. J. Thorac. Oncol.3, 815–818 (2008). ArticlePubMedGoogle Scholar
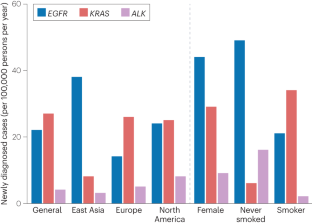
- Li, S. et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br. J. Cancer110, 2812–2820 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kris, M. G. et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA311, 1998–2006 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Zhang, Y. L. et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget7, 78985–78993 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Swanton, C. & Govindan, R. Clinical implications of genomic discoveries in lung cancer. N. Engl. J. Med.374, 1864–1873 (2016). ArticleCASPubMedGoogle Scholar
- AACR Project GENIE Consortium et al. AACR Project Genie: Powering precision medicine through an international consortium. Cancer Discov.7, 818–831 (2017). ArticleGoogle Scholar
- Dogan, S. et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Cancer Res.18, 6169–6177 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Etzel, C. J., Amos, C. I. & Spitz, M. R. Risk for smoking-related cancer among relatives of lung cancer patients. Cancer Res.63, 8531–8535 (2003). CASPubMedGoogle Scholar
- Matakidou, A., Eisen, T. & Houlston, R. S. Systematic review of the relationship between family history and lung cancer risk. Br. J. Cancer93, 825–833 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Coté, M. L. et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur. J. Cancer48, 1957–1968 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Mucci, L. A. et al. Familial risk and heritability of cancer among twins in Nordic countries. J. Am. Med. Assoc.315, 68–76 (2016). ArticleCASGoogle Scholar
- Caron, O., Frebourg, T., Benusiglio, P. R., Foulon, S. & Brugières, L. Lung adenocarcinoma as part of the Li–Fraumeni syndrome spectrum: preliminary data of the LIFSCREEN randomized clinical trial. JAMA Oncol.3, 1736–1737 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Gazdar, A. et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J. Thorac. Oncol.9, 456–463 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet.49, 1126–1132 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Klein, R. J. & Gümüş, Z. H. Are polygenic risk scores ready for the cancer clinic? – a perspective. Transl. Lung Cancer Res.11, 910–919 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hung, R. J. et al. Assessing lung cancer absolute risk trajectory based on a polygenic risk model. Cancer Res.81, 1607–1615 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dai, J. et al. Identification of risk loci and a polygenic risk score for lung cancer: a large-scale prospective cohort study in Chinese populations. Lancet Respir. Med.7, 881–891 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence – SEER Research Data, 8 Registries, Nov 2021 Sub (1975-2020) – Linked To County Attributes – Time Dependent (1990–2020) Income/Rurality, 1969–2020 Counties. https://seer.cancer.gov/statistics-network/explorer (National Cancer Institute, 2023).
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Mortality – All COD, Aggregated With State, Total U.S. (1969-2020), Katrina/Rita Population Adjustment. https://seer.cancer.gov/statistics-network/explorer (National Cancer Institute, 2022).
- Paci, E. et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax72, 825–831 (2017). ArticlePubMedGoogle Scholar
- Infante, M. et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am. J. Respir. Crit. Care Med.191, 1166–1175 (2015). ArticlePubMedGoogle Scholar
- Saghir, Z. et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax67, 296–301 (2012). ArticlePubMedGoogle Scholar
- Becker, N. et al. Lung cancer mortality reduction by LDCT screening – results from the randomized German LUSI trial. Int. J. Cancer146, 1503–1513 (2020). ArticleCASPubMedGoogle Scholar
- de Koning, H. J. et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med.382, 503–513 (2020). ArticlePubMedGoogle Scholar
- Aberle, D. R. et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med.365, 395–409 (2011). ArticlePubMedGoogle Scholar
- Krist, A. H. et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. J. Am. Med. Assoc.325, 962–970 (2021). ArticleGoogle Scholar
- Canadian Task Force on Preventive Health Care. Recommendations on screening for lung cancer. Can. Med. Assoc. J.188, 425–432 (2016). ArticleGoogle Scholar
- Oudkerk, M. et al. European position statement on lung cancer screening. Lancet Oncol.18, e754–e766 (2017). ArticlePubMedGoogle Scholar
- UK National Screening Committee. Adult screening programme: lung cancer. GOV.UKhttps://view-health-screening-recommendations.service.gov.uk/lung-cancer/ (2022).
- Bach, P. B. et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA307, 2418–2429 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Japan Radiological Society The Japanese imaging guideline 2013. Japan Radiological Societyhttp://www.radiology.jp/content/files/diagnostic_imaging_guidelines_2013_e.pdf (2013).
- Zhou, Q. et al. China national lung cancer screening guideline with low-dose computed tomography (2018 version) [Chinese]. Zhongguo Fei Ai Za Zhi21, 67–75 (2018). PubMedGoogle Scholar
- Jang, S. H. et al. The Korean guideline for lung cancer screening. J. Korean Med. Assoc.58, 291–301 (2015). ArticleGoogle Scholar
- Triphuridet, N. & Henschke, C. Landscape on CT screening for lung cancer in Asia. Lung Cancer10, 107–124 (2019). PubMedPubMed CentralGoogle Scholar
- Sagawa, M., Nakayama, T., Tanaka, M., Sakuma, T. & Sobue, T. A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of Jpn. J. Clin. Oncol.42, 1219–1221 (2012). ArticlePubMedGoogle Scholar
- dos Santos, R. S. et al. Do current lung cancer screening guidelines apply for populations with high prevalence of granulomatous disease? Results from the first Brazilian lung cancer screening trial (BRELT1). Ann. Thorac. Surg.101, 481–486 (2016). ArticlePubMedGoogle Scholar
- Ministéro Saúde. Protocolos clínicos e diretrizes terapêuticas em oncologia. Ministéro Saúdehttps://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticas-pcdt/arquivos/2014/livro-pcdt-oncologia-2014.pdf (2014).
- Toumazis, I. et al. Cost-effectiveness evaluation of the 2021 US Preventive Services Task Force recommendation for lung cancer screening. JAMA Oncol.7, 1833–1842 (2021). ArticlePubMedGoogle Scholar
- Criss, S. D., Sheehan, D. F., Palazzo, L. & Kong, C. Y. Population impact of lung cancer screening in the United States: projections from a microsimulation model. PLoS Med.15, e1002506 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Kee, D., Wisnivesky, J. & Kale, M. S. Lung cancer screening uptake: analysis of BRFSS 2018. J. Gen. Intern. Med.36, 2897–2899 (2021). ArticlePubMedGoogle Scholar
- Cao, W. et al. Uptake of lung cancer screening with low-dose computed tomography in China: a multi-centre population-based study. EClinicalMedicine52, 101594 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Quaife, S. L. et al. Lung screen uptake trial (LSUT): randomized controlled clinical trial testing targeted invitation materials. Am. J. Respir. Crit. Care Med.201, 965–975 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- National Cancer Institute. Breast, cervical, and colorectal cancers – early detection summary table. NIHhttps://progressreport.cancer.gov/tables/breast-cervical (2022).
- Jonnalagadda, S. et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer77, 526–531 (2012). ArticlePubMedGoogle Scholar
- Carter-Harris, L., Ceppa, D. P., Hanna, N. & Rawl, S. M. Lung cancer screening: what do long-term smokers know and believe? Health Expect.20, 59–68 (2017). ArticlePubMedGoogle Scholar
- Gesthalter, Y. B. et al. Evaluations of implementation at early-adopting lung cancer screening programs: lessons learned. Chest152, 70–80 (2017). ArticlePubMedGoogle Scholar
- Medicare. Lung cancer screenings. Medicare.govhttps://www.medicare.gov/coverage/lung-cancer-screenings (2023).
- Carter-Harris, L. & Gould, M. K. Multilevel barriers to the successful implementation of lung cancer screening: Why does it have to be so hard? Ann. Am. Thorac. Soc.14, 1261–1265 (2017). ArticlePubMedGoogle Scholar
- Modin, H. E. et al. Pack-year cigarette smoking history for determination of lung cancer screening eligibility. comparison of the electronic medical record versus a shared decision-making conversation. Ann. Am. Thorac. Soc.14, 1320–1325 (2017). ArticlePubMedGoogle Scholar
- American Lung Association. State of lung cancer. American Lung Associationhttps://www.lung.org/research/state-of-lung-cancer (2022).
- Jia, Q., Chen, H., Chen, X. & Tang, Q. Barriers to low-dose CT lung cancer screening among middle-aged Chinese. Int. J. Environ. Res. Public Health 202017, 7107 (2020). Google Scholar
- Novellis, P. et al. Lung cancer screening: who pays? Who receives? The European perspectives. Transl. Lung Cancer Res.10, 2395–2406 (2021). ArticlePubMedPubMed CentralGoogle Scholar
- Crosbie, P. A. et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax74, 405–409 (2019). ArticlePubMedGoogle Scholar
- Verghese, C., Redko, C. & Fink, B. Screening for lung cancer has limited effectiveness globally and distracts from much needed efforts to reduce the critical worldwide prevalence of smoking and related morbidity and mortality. J. Glob. Oncol.4, 1–7 (2018). PubMedGoogle Scholar
- Shankar, A. et al. Feasibility of lung cancer screening in developing countries: challenges, opportunities and way forward. Transl. Lung Cancer Res.8, S106–S121 (2019). ArticleCASPubMedPubMed CentralGoogle Scholar
- Fitzgerald, R. C., Antoniou, A. C., Fruk, L. & Rosenfeld, N. The future of early cancer detection. Nat. Med.28, 666–677 (2022). ArticleCASPubMedGoogle Scholar
- Liu, M. C., Oxnard, G. R., Klein, E. A., Swanton, C. & Seiden, M. V. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol.31, 745–759 (2020). ArticleCASPubMedGoogle Scholar
- Hubbell, E., Clarke, C. A., Aravanis, A. M. & Berg, C. D. Modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiol. Biomark. Prev.30, 460–468 (2021). ArticleCASGoogle Scholar
- Hackshaw, A. et al. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br. J. Cancer125, 1432–1442 (2021). ArticlePubMedPubMed CentralGoogle Scholar
- Mouritzen, M. T. et al. Nationwide survival benefit after implementation of first-line immunotherapy for patients with advanced NSCLC – real world efficacy. Cancers13, 4846 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Smeltzer, M. P. et al. The International Association for the Study of Lung Cancer global survey on molecular testing in lung cancer. J. Thorac. Oncol.15, 1434–1448 (2020). ArticlePubMedGoogle Scholar
- Febbraro, M. et al. Barriers to access: global variability in implementing treatment advances in lung cancer. Am. Soc. Clin. Oncol. Educ. Book42, 1–7 (2022). PubMedGoogle Scholar
- US Environmental Protection Agency. Learn about impacts of diesel exhaust and the Diesel Emissions Reduction Act (DERA). EPAhttps://www.epa.gov/dera/learn-about-impacts-diesel-exhaust-and-diesel-emissions-reduction-act-dera (2023).
- Ervik, M. et al. Global Cancer Observatory: Cancer Over Time (International Agency for Research on Cancer, accessed 1 May 2022); https://gco.iarc.fr/overtime.
- Soda, M. et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature448, 561–566 (2007). ArticleCASPubMedGoogle Scholar
- Shaw, A. T. et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol.27, 4247–4253 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kim, H. R. et al. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer118, 729–739 (2012). ArticleCASPubMedGoogle Scholar
Author information
Authors and Affiliations
- Division of Endocrinology, Diabetes, and Bone Diseases, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA Amanda Leiter
- Division of Hematology and Oncology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA Rajwanth R. Veluswamy
- Division of General Internal Medicine, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA Juan P. Wisnivesky
- Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA Rajwanth R. Veluswamy
- Amanda Leiter
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
R.V. has acted as an adviser and/or consultant to AstraZeneca, Beigene, BerGenBio, Bristol-Myers Squibb, Merck, Novartis, Novocure and Regeneron, and has received research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and Onconova Therapeutics. J.P.W. has acted as an adviser and/or consultant to Atea, Banook, PPD and Sanofi and has received research grants from Arnold Consultants, Regeneron and Sanofi. A.L. declares no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks D. Christiani and the other, anonymous reviewers for the peer-review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leiter, A., Veluswamy, R.R. & Wisnivesky, J.P. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol 20, 624–639 (2023). https://doi.org/10.1038/s41571-023-00798-3
- Accepted : 20 June 2023
- Published : 21 July 2023
- Issue Date : September 2023
- DOI : https://doi.org/10.1038/s41571-023-00798-3
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable link
Sorry, a shareable link is not currently available for this article.
Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative









