Biuret Test: Principle, Procedure, and Uses
Protein analysis is done for various reasons; for example, in clinical laboratories, it is used for determining disease by analyzing serum proteins. The biuret test is one of the methods of protein analysis.
The biuret test is a colorimetric test that helps detect specific proteins or peptide bonds in given analytes. It is followed by spectrophotometry for quantification.
The test requires the use of a biuret reagent. This reagent is a solution that consists of hydrated copper (II) sulfate, sodium hydroxide, and potassium sodium tartrate.
The use of copper (II) ions present in the biuret reagent results in the formation of purple coloration if peptides are present. The intensity of the purple color is measured using a spectrophotometer.
Table of Contents
Principle of Biuret Test
Biuret test requires testing the analytes with biuret reagents. The reagent is a mixture of potassium sodium tartrate (KNaC4H4O6 or C4H4KNaO6), copper (II) sulfate or cupric sulfate (CuSO4), and sodium hydroxide (NaOH).
Sodium hydroxide makes the solution alkaline, and potassium sodium tartrate is the chelating agent. The potassium sodium tartrate helps stabilize the cupric ions in the mixture and maintains the alkaline solution’s solubility.
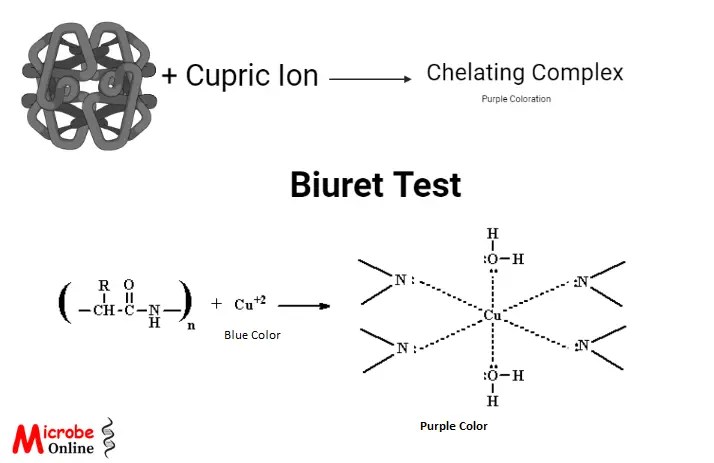
The four nitrogen atoms present in the protein peptides bind to the reagent’s copper (II), resulting in a change of cupric ions to cuprous ions and displacement of peptide hydrogen under alkaline conditions.
Nitrogen binding to cupric ions also results in donating the lone pairs of an electron from nitrogen to the copper ions to form coordinated covalent bonds. The coordinate covalent bond with cupric ions forms a chelate complex that absorbs light with a wavelength of 540 nm, which imparts purple color. Hence, the formation of purple color indicates the presence of proteins in the analyte.
The test depends on the peptide bonds instead of the presence of amino acids in the sample, so it can help measure the protein concentration in whole tissue samples. Proteins purified using ammonium sulfate ((NH4)2SO4) may give false positive results due to nitrogen in ammonia.
Reagents and Materials Required
The reagents and materials required for the biuret test are as follows:
- The reagent required for performing a the test is biuret reagent. The biuret reagent is prepared by mixing 1% solution of CuSO4 (1 gm CuSO4 in 100 ml water) and 1.2 grams of potassium sodium tartrate. 10 ml 10% solution of NaOH (10 gm NaOH in 100 ml water) is added to the above mixture, known as a biuret reagent. The solution is blue in color due to the presence of the CuSO4.
- Other equipment required for the test are test tubes, a dropper or a pipette, a test tube holder, and a stand.
Procedure of Biuret Test
- Take three clean and dry test tubes.
- In the first tube, add 1-2 ml test sample. Likewise, add 1-2 ml of egg albumin in the second one; in the third tube, add 1-2 ml of distilled water. The egg albumin is a positive control, whereas distilled water is a negative control for this test.
- Then, add 1-2 ml biuret reagent in all three tubes.
- After that, properly shake all the tubes to mix the reagent and samples/analytes. Then, let the mixture in the tubes stand for at least 5 minutes.
- Finally, observe the color change.
Precautionary measures
- Use test tube holders when holding the tubes with a solution.
- When preparing biuret reagent, handle NaOH carefully as it is a strong base that might cause corrosion when exposed to the skin.
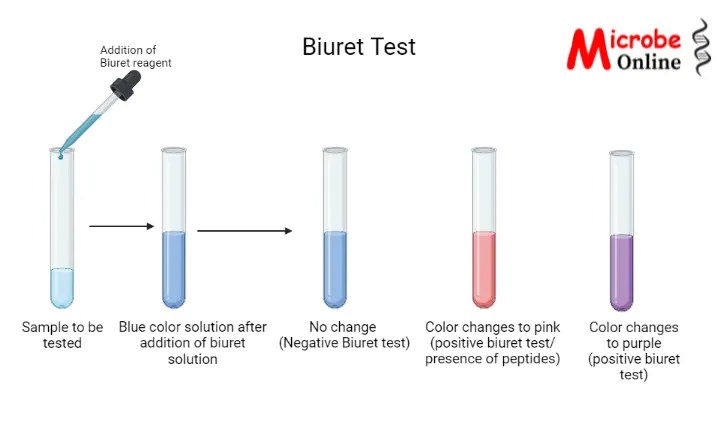
Result Interpretation
The results of the biuret test are interpreted as follows:
Uses of Biuret Test
- The test helps in determining the type of proteins in unknown samples.
- It is used for quantification of protein by using a spectrophotometer alongside.
- It can help determine proteins in the urine, CSF, and other body fluids.
- The test helps determine the presence of specific proteins during food analysis.
Advantages of Biuret Test
- The test is simple and inexpensive.
- It can detect nitrogen from only peptide bonds.
- Very few components interfere with the test.
- The color is stable, so it causes less deviation.
- It is also a rapid test.
- It can detect proteins with at least four peptide bonds.
Disadvantages of Biuret Test
- The presence of amino acid histidine can give false positive results because there is a presence of nitrogen.
- If the buffer used to purify proteins has ammonium and magnesium salts, it can hinder the test.
- The presence of carbohydrates and fats can also hinder the test.
- This test alone cannot help in quantifying the protein in the sample; spectrophotometric analysis is required for quantification.
- Its sensitivity is lower than the Folin Lowry test.
- Only soluble proteins are helpful in this test, and different proteins give different colors, so standardization of colors is required for known proteins.
- A. Bianchi-Bosisio, PROTEINS | Physiological Samples, Editor(s): Paul Worsfold, Alan Townshend, Colin Poole, Encyclopedia of Analytical Science (Second Edition), Elsevier, 2005, Pages 357-375, ISBN 9780123693976, https://doi.org/10.1016/B0-12-369397-7/00494-5. (https://www.sciencedirect.com/science/article/pii/B0123693977004945)
- Mohanlal Sukhadia University. (n.d.). Biuret test – Mohanlal Sukhadia University. https://www.mlsu.ac.in/econtents/2207_Biuret%20test.pdf.



